Plenary Poster 30th Australian and New Zealand Bone and Mineral Society Annual Scientific Meeting 2020
The Use of Denosumab in Central Giant Cell Granuloma: 5-year Institutional Experience (#18)
Introduction: Central giant cell granuloma (CGCG) is a rare, benign tumour of the jaw typically occurring in children and young adults, characterised by osteoclast-like multinucleated giant cells and local RANKL overexpression by mononucleated stromal cells. Traditional management is surgery but with potential significant morbidity and recurrence.
Denosumab targets RANKL, inhibits osteoclastic bone resorption and when given 120mg monthly is effective in the management of a related condition, namely giant cell tumour of the bone. Experience in CGCG is limited to case reports and small case series. Optimal dosing and monitoring remain unclear.
Aims: To review treatment regimen, clinical, biochemical and imaging changes, and adverse effects during and post-denosumab for CGCG.
Methods: Patients with histologically confirmed CGCG managed by Westmead Hospital Oral-Maxillofacial Surgery and referred to Endocrinology for denosumab 2015-2020 were identified. Baseline data was obtained from medical records and external providers including demographics, comorbidities, symptoms, investigations (imaging, biochemistry, parathyroid hormone, 25-hydroxyvitamin D, bone turnover markers). Clinical features, investigations and adverse effects during and post-cessation of denosumab were obtained.
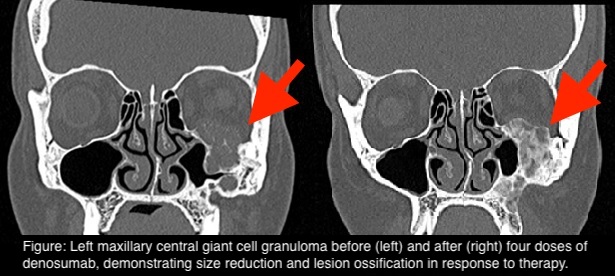
Results: We identified seven cases of CGCG treated with denosumab, the largest adult series in literature to our knowledge. Four (57%) were male. Three were mandibular, three were maxillary, one involved both. Three had multiple lesions. Mean 25-hydroxyvitamin D pretreatment was 69±11.8 nmol/L. Mean age at denosumab commencement was 24±9.0 years. Three were treatment-naïve, three had intralesional steroids, two had previous surgery. Denosumab was effective in reducing symptoms and size after 5±3.4 doses. Five ceased after 15±6.8 doses but three (60%) experienced recurrence 13±4.2 months post-cessation. One had rebound hypercalcaemia post-cessation. There were no cases of osteonecrosis of the jaw.
Conclusion: Denosumab has demonstrated efficacy for CGCG and is promising neoadjuvant therapy for improving ability to provide surgical intervention. Long-term safety of high dose denosumab in younger subjects needs evaluation.